KIT GENE - WHITE MASKING AND WHITE SPOTTING (PIEBALD) & STUDY OF KIT GENE EDITING
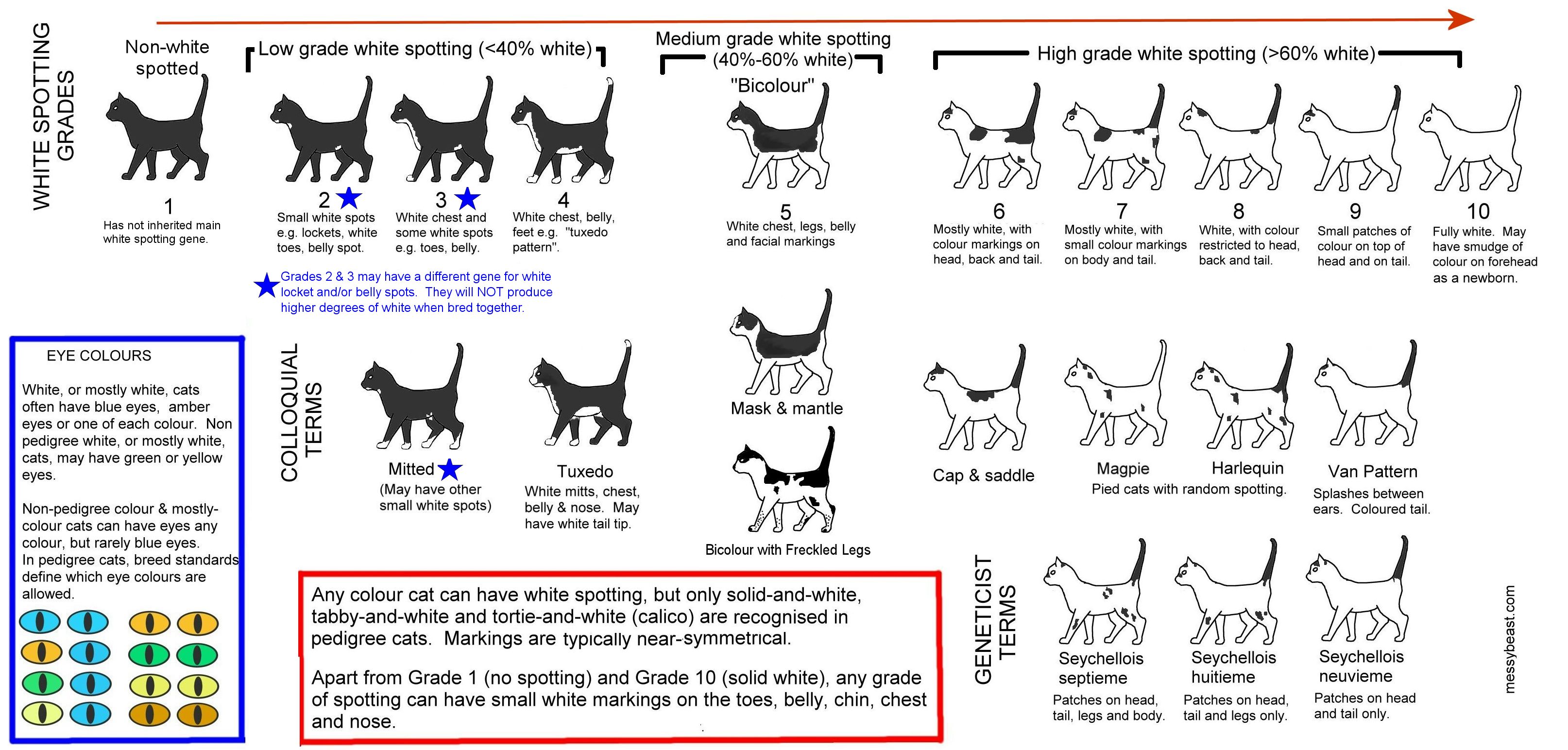
"White Masking" and "White Spotting" and White gloves" are alleles of the same gene (called KIT). Genes are inherited in pairs so there are various combinations e.g. Wws, Wwg, wswg, wwg etc where a cat can be homozygous or heterozygous for a particular degree of white. The order of dominance is W >> ws >> w >> wg the more dominant gene of each pair determines the visible pattern.
W = solid white (W/W is homozygous solid white, W/ws, W/w & W/wg are heterozygous solid white)
ws = white spotting (ws/ws is homozygous white spotting, ws/w & ws/wg are heterozygous white spotting)
w = non-white (w/w is homozygous non-white, w/wg is heterozygous non-white; w+ is also used = "wild-type")
wg = white gloves (has to be wg/wg to give white gloves)
There may possibly be other spotting mutations for white gloves (Birman), white mitts (Ragdoll), white throat locket, white brisket spots and there is not yet a symbol for the Altai version of white spotting. So-called "recessive white" is part of the albinism series, a different gene. Not all white spotting is due to KIT gene mutations. Other genes can also have mutations causing white markings e.g. vitiligo.
PHENOTYPES OF KIT GENE MUTATIONS
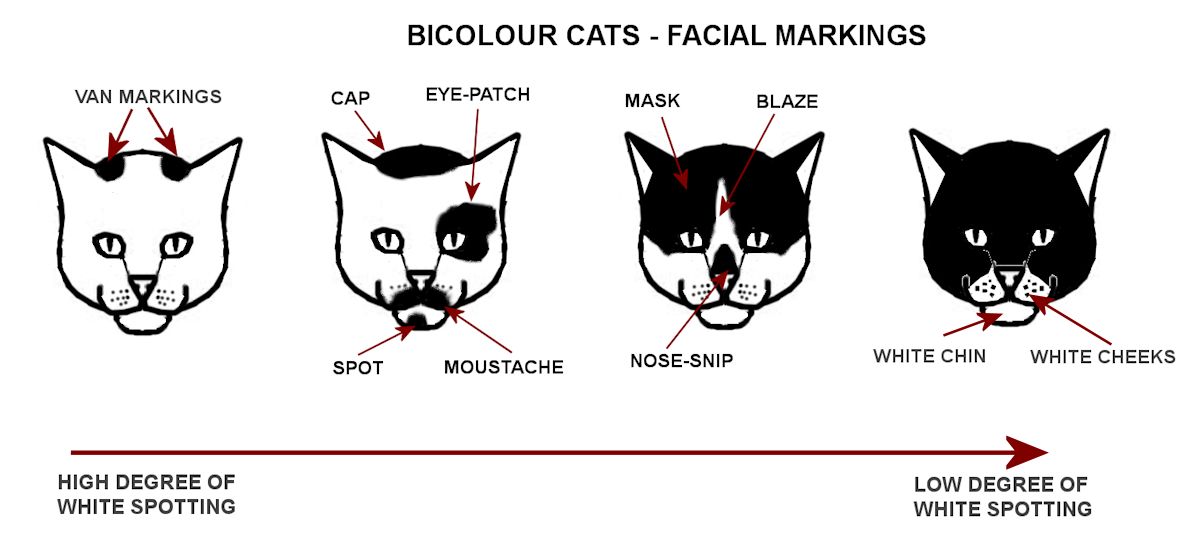
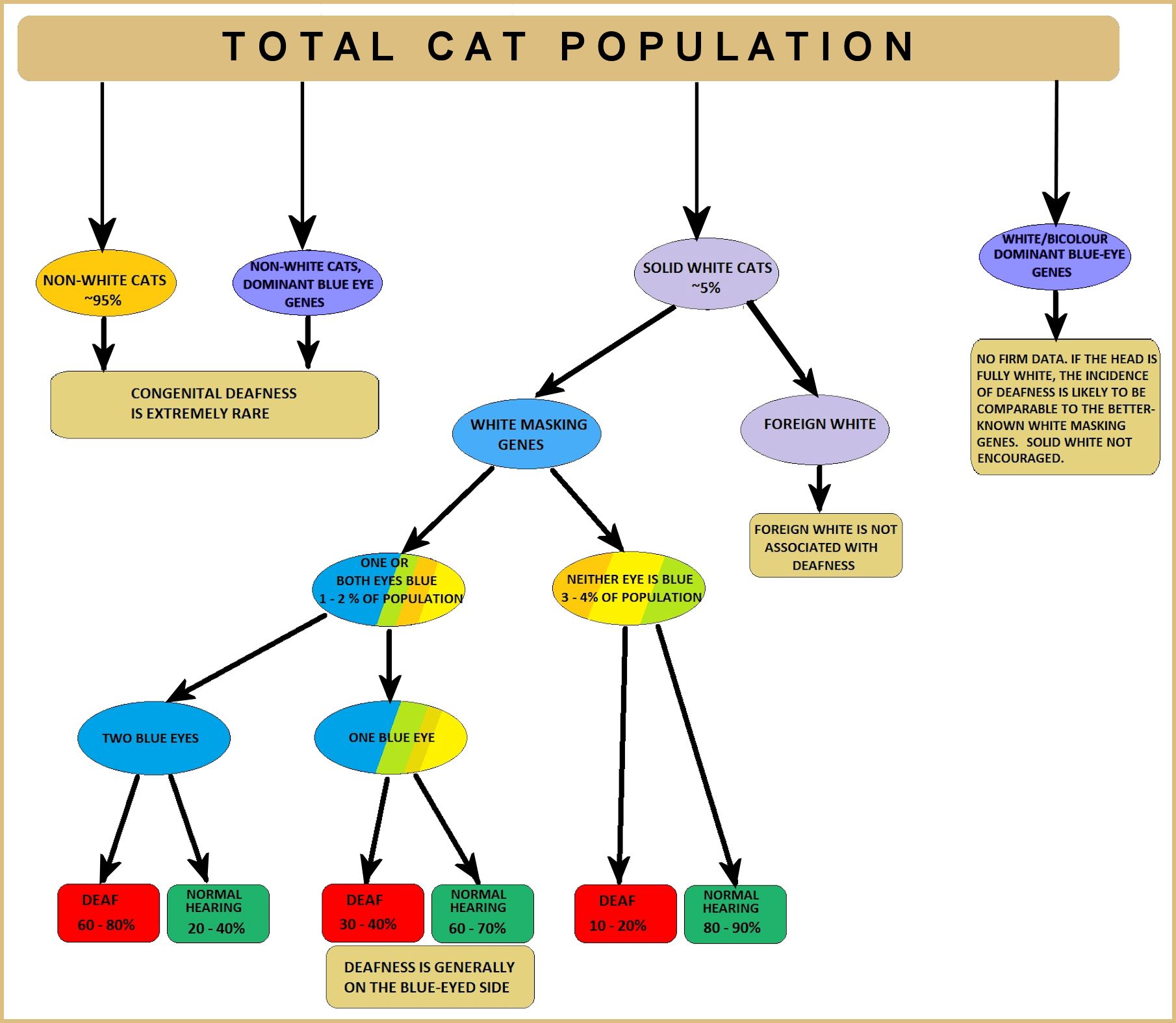
White masking (W) produces an entirely white coat, with pink nose and paw pads. Only one copy is needed to produce solid white. One or both eyes may be blue and the cat may be deaf on the blue-eyed side(s) because the eye tissue and ear tissue are close together in the embryo and melanin is required for proper function of the inner ear. White spotting creates white patches on the body because the fur and underlying skin lack pigment where the white spotting gene is active. If the white spotting gene acts on the embryo cells that give rise to the eyes, this gives blue eyes. If it acts on the cells that create the skin around the eyes, but not on the embryonic eye cells, the eyes are orange, yellow, green etc. The eyes can be different colours. Sometimes an odd-eyed cat is deaf on the blue-eyed side because the embryo ear tissue cells are also affected; this is more likely in cats with a lot of white on the face.
White spotting (ws) patterns are extremely variable. A cat with a single copy of ws can have a small or a large amount of white. Anecdotally, cats with 2 copies of ws tend to have more white, and may even be solid white. Selective breeding can fix the amount of white because other genes, known as polygenes, can influence colour distribution e.g. some breeds have been fixed for gloves or mitts.

KIT GENE MUTATIONS, DEAFNESS IN WHITE-MARKED CATS
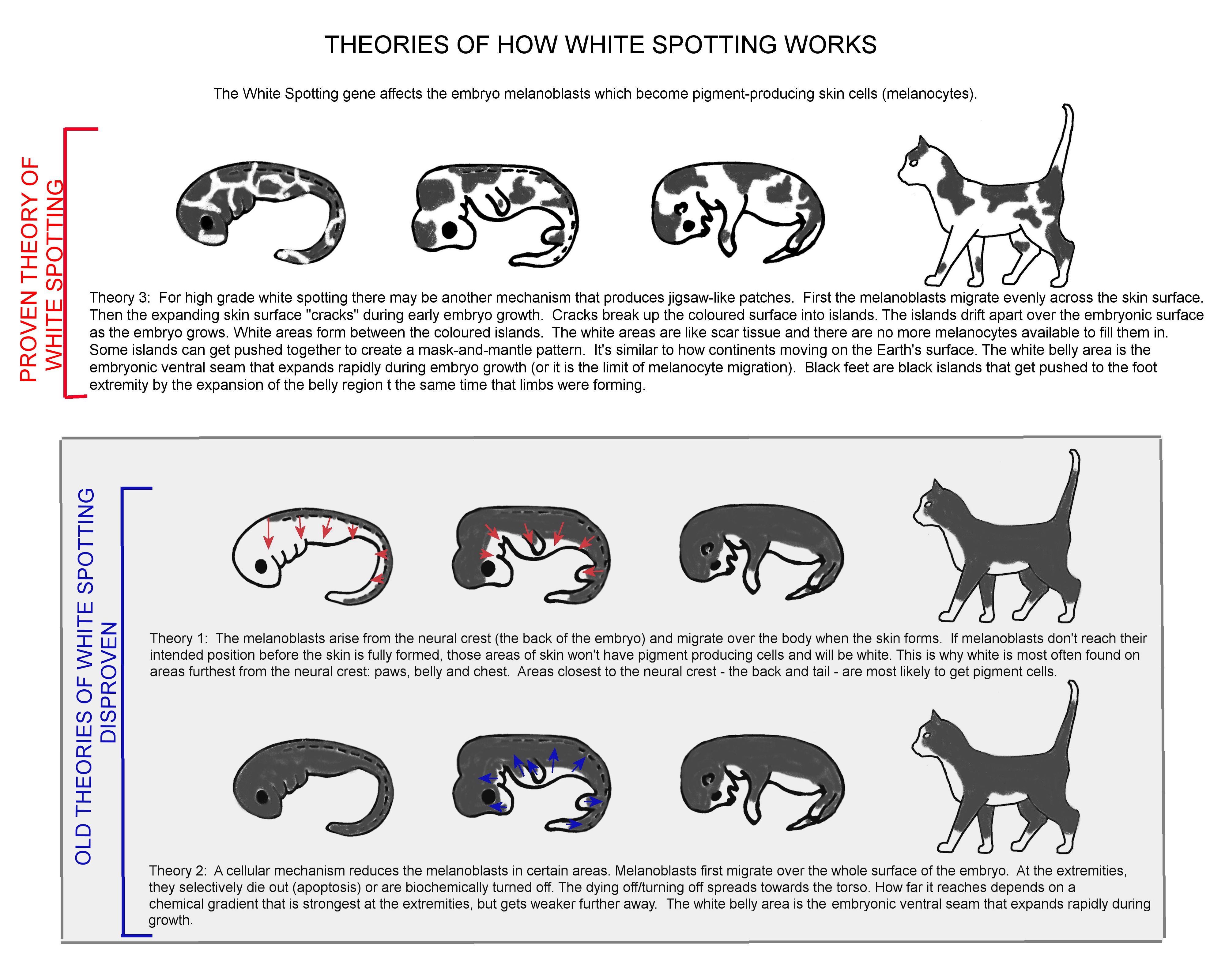
Dominant White (W) and White Spotting (ws) result from the insertion of an entire common 'feline endogenous retrovirus' (FERV1) in the KIT gene. A full 7125 base-pair FERV1 insertion results in White Spotting. A FERV1 long terminal repeat (LTR) (a partial insertion) results in Dominant White. Only 1 copy of the full insertion is required for the White Spotting colour pattern to appear and the other allele is wild-type (non-white). It disrupts migration of melanocytes to certain patches in the skin, hence the white patches. The gene(s) controlling the actual pattern of White Spotting isnt yet known. 1 or 2 copies of the partial insertion produces solid white regardless of the other allele. It appears that cats with 2 copies of the mutation are more likely to be deaf than cats with one copy.
The deafness in solid white cats and some white spotted cats (with a high degree of white around the eyes and ears) is due to a reduction in the population and survival of melanoblast stem cells, which in addition to creating pigment-producing cells, develop into a variety of neurological cell types. The deafness is caused by degeneration of the cochlea and saccule tissues immediately after birth due to the lack of melanocytes in the inner ear (albino cats have normal melanocytes in the inner ear).
The linkage of white markings and deafness is found in Waardenburg syndrome is several mammal species. In humans these are associated with mutations in EDN3, EDNRB, MITF, PAX3, SNAI2, and SOX10. Others consider any form of white marking associated with deafness to be a form of Waardenburg syndrome (which would then include the KIT gene).
OTHER ROLES OF THE KIT GENE
The full name of the KIT gene is "v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog." It is a proto-oncogene (helps cell growth and cell division, help cells stay alive and plays a role in programmed cell death) and its products play a role in foetal development. It is expressed in haemopoietic stem cells, mast cells, intraepithelial lymphocytes, germ cells, melanocytes, some muscle cells, and the product of the gene has a role as a growth factor receptor in those cells. A proto-oncogene can mutate into an oncogene which is involved in tumours.
The KIT gene is pleiotropic and has multiple roles in embryo development, not just the production of skin pigment. It also plays a role in stem cells and progenitor cells, in muscle cells and in connective tissue cells in the gastrointestinal tract.
The KIT gene encodes the mast/stem cell growth factor tyrosine kinase receptor on the cell surface; this receptor detects signals from outside of the cell which triggers processes inside the cell. KIT mutations cause partial loss of receptor function or abnormal activation of downstream signalling which can result in tumours. The most visible sign of KIT mutations is white markings due to its effect on pigment producing cells.
SOURCES FOR GENERAL KIT GENE/WHITE SPOTTING INFORMATION
EFFICIENT GENERATION OF CLONED CATS WITH ALTERED COAT COLOUR BY EDITING OF THE KIT GENE
Coat colour is an important characteristic of cat breeds and affects their market popularity/adoptability and perceived personality [Carini RM, Sinski J, Weber JD. Coat color and cat outcomes in an urban U.S. Shelter. Animals 2020;10(10):1720.]. The Chinese Li Hua (Dragon Li) has brown or orange mackerel tabby coats. Chartreux cats are solid blue-grey and Turkish Angoras are most popular in the solid white colour. GM methods could produce new commercially viable colours [note: western breeders may consider this unethical, but cat ownership in China appears to have a more commercial basis and some whited patterns are considered auspicious].
The KIT gene codes the receptor tyrosine kinase of Stem Cell Factor (SCF), an essential regulator of the migration and differentiation of melanin producing cells. KIT is pleiotropic (affects multiple functions) and also stimulates multiple downstream signalling pathways controlling important cellular processes [Randall VA, Jenner TJ, Hibberts NA, De Oliveira IO, Vafaee T. Stem cell factor/c-Kit signalling in normal and androgenetic alopecia hair follicles. J Endocrinol 2008;197(1):1123; Pham DDM, Guhan S, Tsao H. KIT and melanoma: biological insights and clinical implications. Yonsei Med J 2020;61(7):56271].
To examine whether editing the KIT gene could create a new coat colour in cats, the authors deleted exon 17 of KIT in Feline Embryonic Fibroblasts (FEFs) and used these to create genetically modified (GM) clones of Chinese Li Hua cats. All cats used in this study were housed at the Henan Liosio Biotechnology Co., Ltd with spacious living quarters, free access to food and water, toys, climbing and scratching posts etc.
Feline Embryonic Fibroblasts were isolated from two 40-day-old Li Hua cat embryos removed from the mother by caesarean section. DNA was extracted from the foetal skin cells and edited to delete exon 17 of the KIT gene. The edited cells were transferred into donor egg cells from the ovaries of cats spayed at local vet clinics. 131 GM embryos were transplanted into 4 surrogates. This is approx 30 per surrogate because other studies have shown that few cloned embryos (both GM and non-GM) reach full term. All surrogates carried their pregnancies to term. 8 GM kittens were born, of which 6 were born alive and 2 were stillborn. 8/131 = 6% survival rate of implanted eggs (considered a high efficiency rate in cats!). 6/8 = 75% survival of full term GM kittens. None of the live-born kittens survived to adulthood. There were also three foetuses that had died at different developmental stages. [It would have been interesting to know the skin colours/patterns of these, but this was not mentioned.]
DNA analysis of the GM kittens and dead foetuses was performed on umbilical cord cells. The tissues of dead kittens (2 stillborn, none of the survivors reached adulthood) and foetuses were tested for the main viral pathogens. Some were found to be infected with Feline Herpesvirus which may have caused their early death. Early death may also be due to incomplete epigenetic reprogramming in cloned embryos, which affects the longer-term health and survival of cloned animals. A possible cause of pre-natal death was the use of donor eggs from ovaries removed during spay surgeries at vet clinics [deterioration during transport/storage, rejection by the surrogate].
KIT gene lacking exon 17 is given as "KIT D17" below. Wild-type (unedited) KIT is shown as KIT+. The authors give a tab le showing how many base pairs are affected. This summary just notes whether there was full deletion, partial deletion or wild type.
Analysis of the dead foetuses implied that complete lack of KIT exon 17 (homozygous KIT D17/KIT D17) could be lethal.
Four kittens (#1, #6, #7, #8) were homozygous wild-type KIT (KIT+/KIT+).
Four kittens (#2, #3, #4, #5) were heterozygous for edited KIT genes, but they did not have identical edits. They were heterozygous for different mutations.
Heterozygous kitten #2 was KIT D17/KIT+ i.e. KIT exon 17 fully deleted on one allele and normal KIT gene on the other allele, allowing investigation of the function of KIT in feline coat colour.
Heterozygous kitten #5 was KIT D17/KIT+ i.e. partial deletion of KIT exon 17 on one, and normal KIT gene on the other allele.
Heterozygous kitten #3 had different edits on each allele i.e. partial deletions of KIT exon 17 on each allele.
Heterozygous kitten #4 had different edits on each KIT allele. KIT exon 17 fully deleted on one allele and partially deleted KIT exon 17 on the other allele, allowing investigation of the function of KIT in feline coat colour.
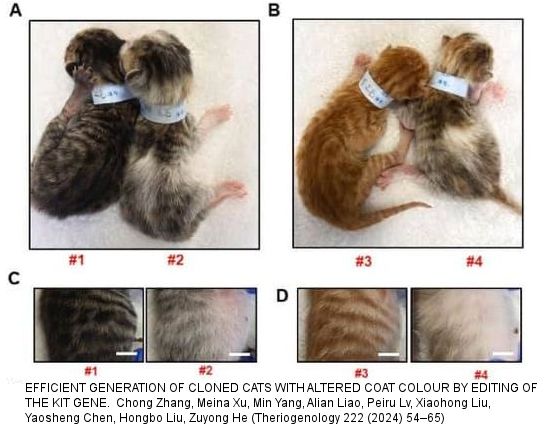
[Note: The photo of #4 looks more like a tortie tabby than an orange tabby, tortie would only be possible if 2 embryos had fused early on. #2 and #4 both had a white patch in the middle of the back. It may also have been due to the KIT D17 edit affecting black pigment more than red pigment.]
Deleting exon 17 of KIT altered the coat colour of heterozygous edited cats. KIT +/+ individuals (#1, #6, #7, #8) had normal brown or orange mackerel tabby coats.
Heterozygous kittens #2 and #4 (each with one copy of KIT exon 17 deleted) were mackerel tabby with almost white lower bellies, white hair on the legs and feet (pink paw pads) and white tail-tips. This suggests that deleting exon 17 of KIT affects both hair and skin pigmentation. Kitten #2 had one normal KIT gene. Kitten 4 had one full deletion and one partial deletion of KIT exon 17; photos show a slightly larger amount of white present [this was the kitten that appears tortoiseshell-tabby with white in photos which makes me suspicious of other issues].
Compared to the normal brown mackerel tabby and normal orange mackerel tabby, the heterozygous GM kittens had a faded appearance. The GM kittens both had a small pale/white patch along the spine from just behind to forelegs to the root of the tail (part the neural crest area). The eumelanin and pheomelanin pigment in the hair shaft from the backs of the GM kittens was reduced compared to the normal kittens. Pigment reduction varied from 15% - 40%, hence the faded appearance. Generally there seemed to be a greater reduction in eumelanin than phaeomelanin. Skin tissue samples showed reduced melanin deposition in the hair follicles of the GM kittens. The deletion of exon 17 of KIT did not affect the development or structure of the hair follicles. Instead it seemed to reduce the number of melanocytes or their production of melanin, particularly along the back. GM kittens #2 and #4 had significantly reduced gp100, a key protein in melanosome production.
The KIT gene plays a role in several cell processes, not just pigmentation. Total lack of KIT exon 17 (homozygous) results in a lack of docking sites for molecules essential for cell survival and for programmed cell death (weakened signalling for these essential cell functions). Lack of one copy of KIT exon 17 (heterozygous) permits partial signalling function which presents as white spotting. Lack of both copies of KIT exon 17 causes foetal death, likely due to the role of KIT exon 17 in producing blood cells.
The researchers conclude that to alter cat coat colour, it is safer to edit gene such as melanocortin 1 receptor gene and tyrosinase, which are involved in melanin synthesis, but unsafe to edit genes that affect multiple developmental processes and cell functions. They also consider that this GM method has great potential for improving companion animal health e.g. preventing osteochondrodysplasia in Scottish Fold cats by editing the known singlebase mutation [Sartore S, Moretti R, Piras LA, Longo M, Chessa S, Sacchi P. Osteochondrodysplasia and the c.1024G>T variant of TRPV4 gene in Scottish Fold cats: genetic and radiographic evaluation. J Feline Med Surg 2023;25(12)]. Eliminating osteochondrodysplasia would be beneficial, but other modification of various cat traits are possible e.g. a miniature cat could be created through precise editing of the paired-like homeodomain factor 1 gene. The GM method described in their paper has great implications for the generation of new breeds with various desired traits, but the cat cloning process is still inefficient and produces few viable kittens.